
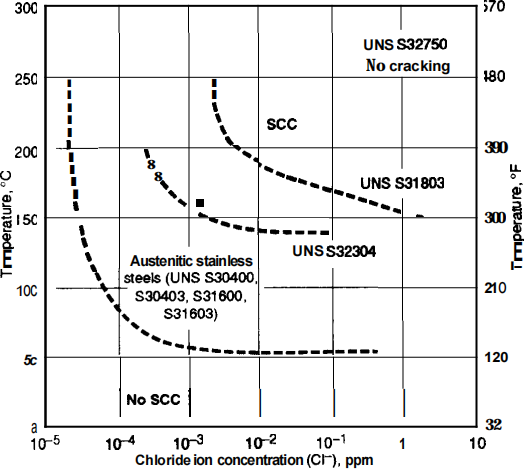
Steel is one of the most commonly used materials in the railway sector due to its strength, versatility and economy. Stainless Steel Use in the Railway Industry Environmental factors such as increased temperatures can also increase the susceptibility of stainless streel grades to stress corrosion cracking. No stainless steel grade can be totally immune to chloride stress corrosion cracking, however the resistance does vary between the grades.
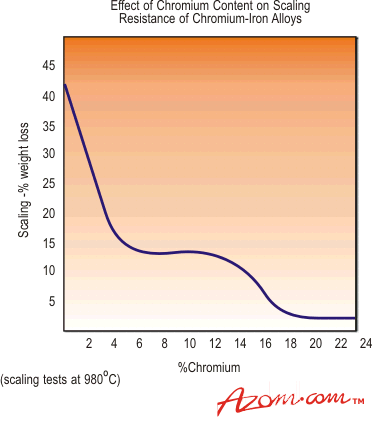
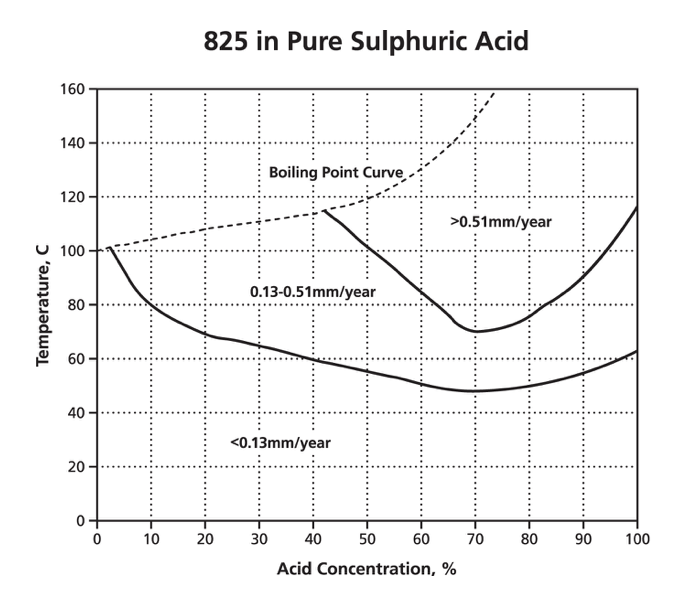
Stainless steel can be alloyed with elements of chromium, nickel and molybdenum to create a more stable oxide layer that is able to protect the metal beneath from chloride corrosion.ĭespite this, the combination of tensile stress and a high-chloride environment can cause stainless steels to crack, in a process known as stress corrosion cracking. Types 316 and 316Ti are considerably more resistant than any of the other chromium-nickel stainless steels to solutions of sulfuric acid. When this layer is exposed to a large concentration of chloride ions it can be destroyed.īecause there are so many corrosion-related issues in standard steel, stainless-steel can be a viable alternative to increase the durability of reinforced concrete structures which are in chloride-rich environment. Early failures in domestic water installations were found due to the use of chloride-containing fluxes which had not been effectively removed after the soldering operation. On the surface of steel, a thin oxide layer forms following exposure to an alkaline medium. Stainless steel joints made with conventional lead-tin solders or with silver-tin alloys are satisfactory where strength is not a major consideration. This lack of durability is exacerbated in severe conditions such as those in contact with oceans, de-icing or other chloride-contaminated environments. 1, the ratios of Cr 2 O 3 /Fe, Cr(OH) 3 /Fe, NiO/Fe and FeO/Fe in the passive film of hydrogen charged samples are lower than those of the uncharged samples. 16 used SIMS to analyze the passive film of 310 stainless steel formed at 0.7 V. Stainless steel is often used in reinforced concrete structures, as despite being one of the most affordable structures, reinforced steel has poor durability. The passive film changes significantly with the presence of hydrogen. Stainless steel is an obvious choice for applications in which corrosion resistance is important, and it is used in a wide variety of applications including in architectural cladding, in railways and in food hygiene. In most common alloys, you’ll find 10 nickel and 2 to 3 molybdenum. Stainless steels of the 316 grade include less chromium often around 16 but increase nickel levels and add molybdenum to the mix. This together with slightly higher stacking fault energy (Schramm and Reed 1975) are the main reasons for better resistance of type 316/316L stainless steel than type 304/304L to stress corrosion cracking in sodium chloride solutions. This is largely due to the large amounts of chromium present. The most popular 304 grade stainless contains 18 chromium and 8 nickel though other alloys exist within the same grade. However, molybdenum itself does not get incorporated in the passive film. Stainless steel is often known as ‘corrosion-resistant steel’ because it doesn’t corrode, stain or rust as much as typical carbon steel.


 0 kommentar(er)
0 kommentar(er)
